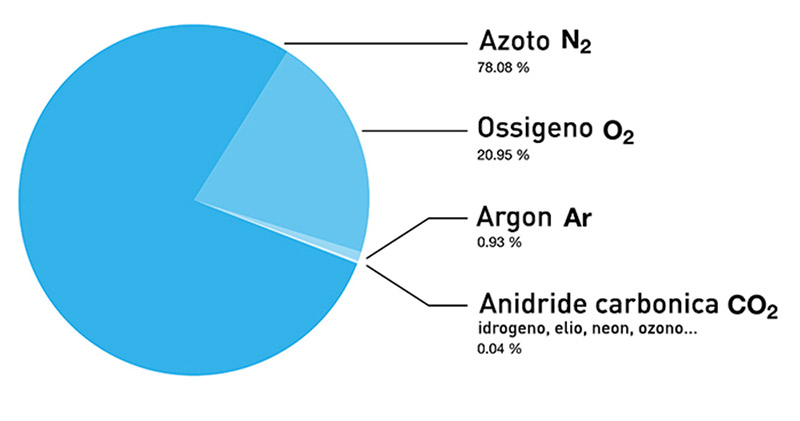
Nitrogen is directly obtained from the air that we breathe every day, which is composed of 78 % nitrogen, 21 % oxygen, and small quantities of carbon dioxide, hydrogen, helium, rare gases (argon, crypton, neon and xenon), water vapor and polluting substances.
WHAT'S THE NITROGEN
HISTORICAL BACKGROUNDS
Nitrogen was discovered around the end of 1700. Until the early 19th Century, scientists believed that air and its main elements, oxygen and nitrogen were “permanent gases” and that could never be transformed into their liquid stage. It was only in 1877 that Louis Cailletet in France and Raoul Pictet in Switzerland produced in their laboratories some bluish drops of liquid air. Later, towards the end of the Century, the German Karl Von Linde and the French Georges Claude developed, independently of each other, techniques to produce liquid air, separating gaseous components on an industrial scale.
These techniques were based on three identical basics:
the first was that the temperature at which a gas liquifies varies depending on the pressure performed on it;
the second is that gases heat up where are being compressed and cool down when they expand. This can be proved by observing a capsule of carbon dioxide which turns common water into soda: the gas is compressed into the capsule at a pressure four or five times greater than the atmospheric pressure, and, once the gas is released in the water of the bottle, the capsule is gelid.
the third rudiment reports that it’s possible to separate a liquid from its gaseous component by boiling it. The volatile part will raise as vapor and can be cooled down by turning it into liquid state in another container. It is the same procedure followed by a farmer that, in order to produce apple brandy, distils alcohol from fermented fruit. The difference between this operation and the work of an air separation plant is only on the temperature. inasmuch the air gases boiling point at atmospheric pressure, which is also where they become liquid, is very low: 183° centigrade below zero for oxygen and 196° centigrade below zero for nitrogen. Those techniques are extremely complex, and science has made big steps forward

Louis Cailletet

Raoul Pictet

Karl Von Linde
The process of air separation plant starts with huge compressors which suck external air and filter water vapor, carbon dioxide and pollutants.
Once purified, the air undergoes to enormous pressure (up to 20 times higher than the atmospheric pressure) to be later expanded and used as refrigerating. Since the compression drastically intensifies the temperature, air circulates through cooling units and it is subjected to further treatments to reach 80° centigrade below zero: at this point of the process, the pressure is almost six times below the norm.
By expanding, this mass of air cools all the rest, that remains subjected to high pressure, up to 160° centigrade below zero. It is in this way that the formation of this bluish liquid called liquid air starts, later followed by several devices that lead to distillation: by boiling it, nitrogen is eliminated and pure oxygen is obtained.

Distillation takes place in stainless steel columns where, there are hundreds of perforated “trays”. The gas rises through the holes while the liquid, overflowing from the trays, pours on the level below. In this way, the gas is enriched with nitrogen and the liquid with oxygen. This process, as well as many others of this industry, is invisible.
Since those operations are made at lower temperatures, they take place within the walls of a cold-box, from which nitrogen can exit both at solid and gaseous state, depending on what and where has to be send.
Nitrogen can be transported under pression in big containers and later stored at liquid state under pression or at atmospheric pression in special containers also called “Dewar”. The isolation of these containers, minimizing the heat flow towards the inside of the container, reduces the loss of nitrogen due to the evaporation of the liquid: with this system, liquid nitrogen (-195,82 °C) can be used as a potent refrigerator.
In order to obtain lower temperatures, it is usually necessary to use liquid helium (268,9 °C).
